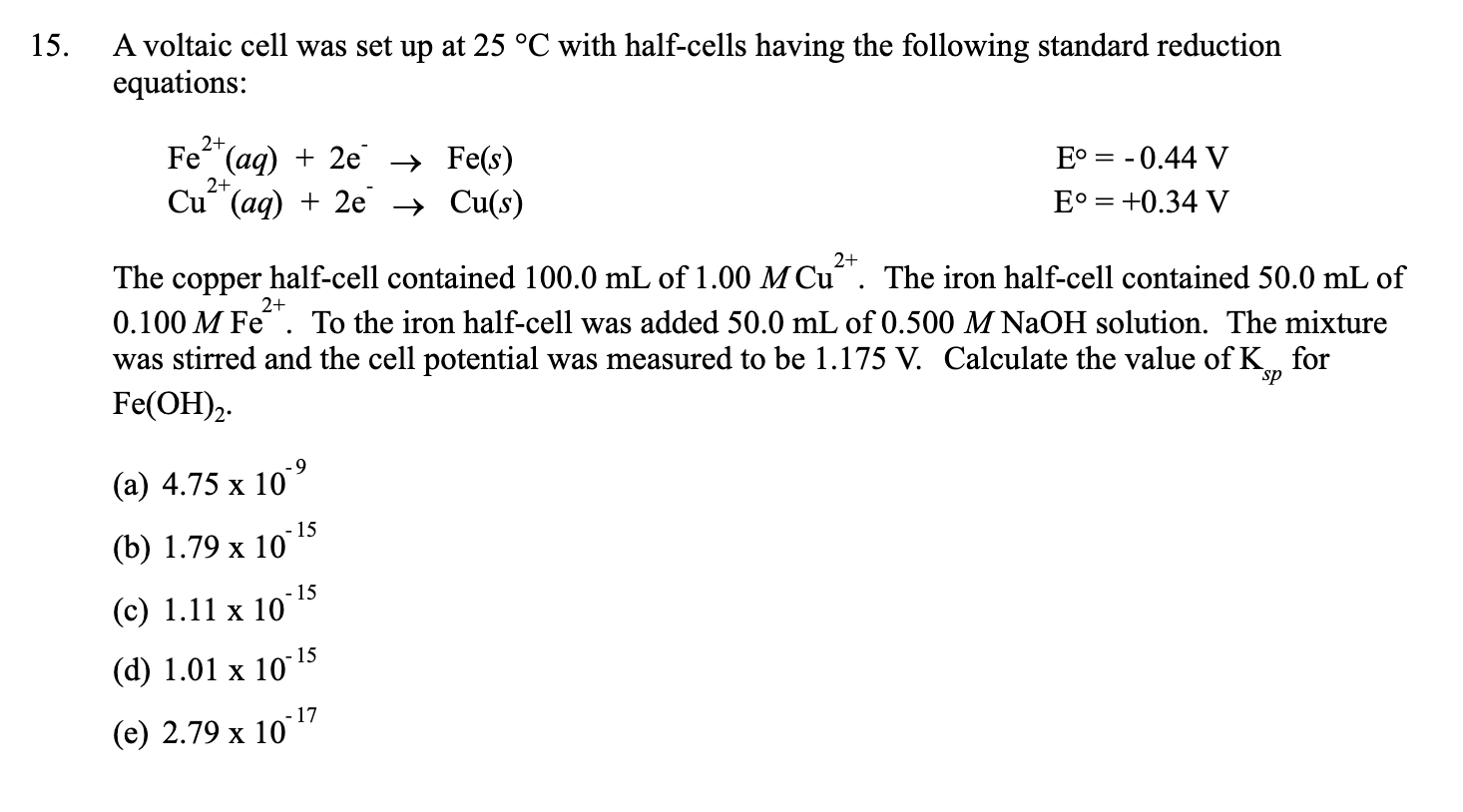
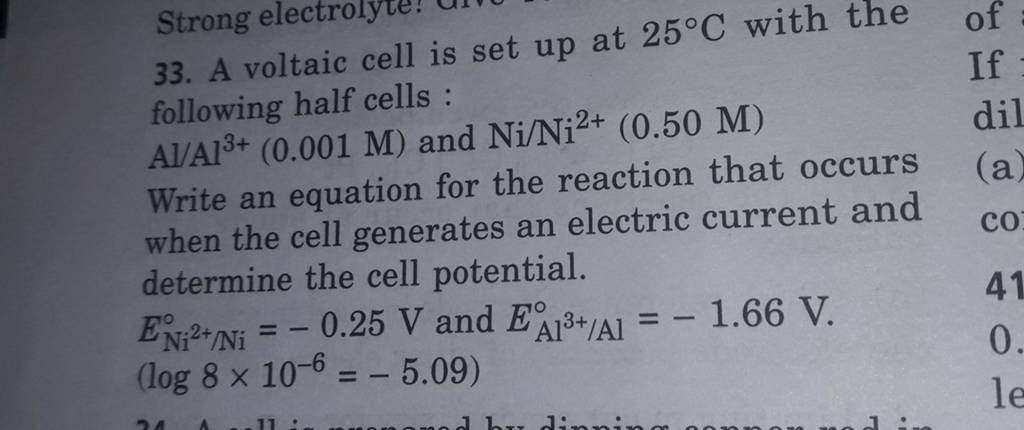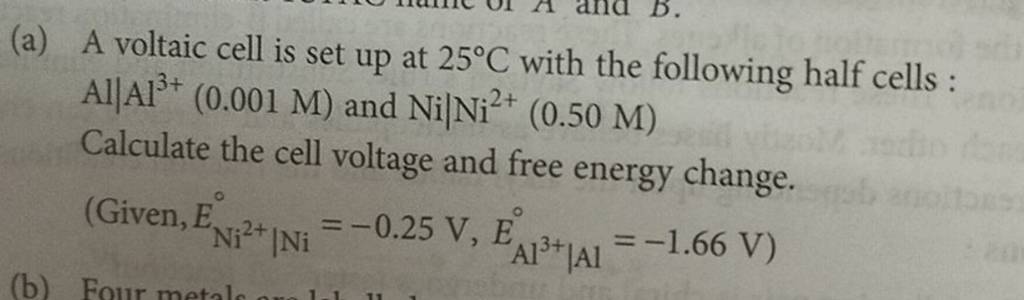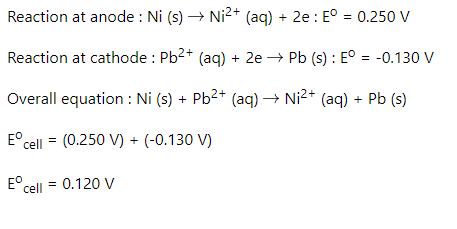SOLVED: A voltaic cell using Pb²⠺/Pb and Ni²⠺/Ni half-cells is set up at standard conditions, and each compartment has a volume of 355 mL. What is the cell potential after
A voltaic cell is set up at 25°C with the following half-cells, Al^3+ (0.001 M) and Ni^2+ (0.50 M). - Sarthaks eConnect | Largest Online Education Community

taic cell is up 25 °C with the following half cells, 9. 20. A voltaic cell is ur A bovlofti eroinsta A13+ and NiNi2+ (0.001 M) (0.5 M) EX13+ / Al = -

3). A voltaic cell is up 25°C with the following half cells : A1+ (0.001 M) and Ni+2 (0.50 M) Write the cell reaction and calculate the cell potential. (Given : Ex+3/4 = -
A voltaic cell is set up at 25°C with the following half cells. Al | Al^3+ (0.0010 M) and Ni^2+ (0.50 M) | Ni - Sarthaks eConnect | Largest Online Education Community
A voltaic cell is set up 25° C with the following half-cells: - Sarthaks eConnect | Largest Online Education Community

SOLVED: A Voltaic cell is set up at 25 °C with the half-cells Al3+(0.0010 M) |Al and Ni2+(0.50 M) |Ni a.Write an equation for the reaction that occurs when the cell generates

PPT - Electrochemical Potential – Non Standard Conditions Variations in Concentration PowerPoint Presentation - ID:3004901
A voltaic cell is setup at 25°C with the half cells Ag^+ (0.001 M) Ag and Cu^2+ (0.10 M) Cu. What should be its cell potential ? - Sarthaks eConnect | Largest Online Education Community
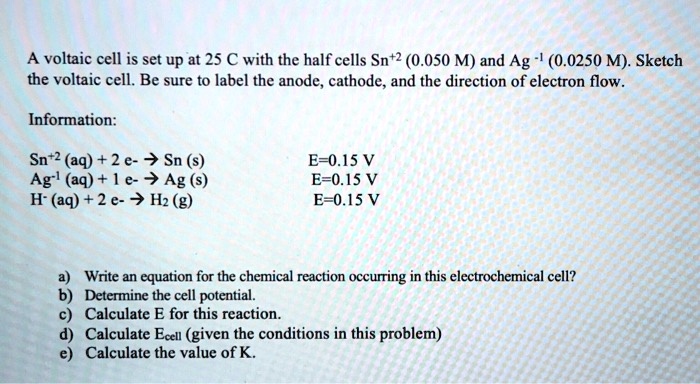
SOLVED: A voltaic cell is set up at 25 € with the half cells Sn+2 (0.050 M) and Ag (0.0250 M): Sketch the voltaic cell. Be sure to label the anode, cathode

A silver concentration cell is set up at 25 degrees C as shown below. The AgCl(s) is in excess in the left compartment. Label the anode and cathode, and describe the direction

b) A voltaic cell is up 25°C with the half-cells, AIAP (0.001 M) and NIIN 0.50 M Write the equation the reaction that occurs when the cell generates an electric current and